Optogenetics illuminates darkness
Using optogenetics, a 58-year-old man with advanced retinitis pigmentosa, who was blind in one eye, was able to perceive light in that eye after treatment.
The Ophtalmology Blog
By Dr. med. Annabelle Eckert, FEBO
At the end of May, we were deeply impressed by the publication of a scientific article in the renowned journal Nature. Using optogenetics, a 58-year-old man with advanced retinitis pigmentosa, who was blind in one eye, was able to perceive light in that eye after treatment. In the better eye, Lux was the best visual acuity.
Optogenetic restoration of vision is a mutation-independent therapy that can be considered for patients with end-stage retinitis pigmentosa with loss of visual acuity. The open-label 1/2a phase of the PIONEER trial (ClinicalTrials.gov identifier: NCT03326336) evaluated the safety and efficacy of this experimental treatment technique in people with advanced stage non-syndromic retinitis pigmentosa. A specific optogenetic vector (GS030-Drug Product (GS030-DP)) was injected. Light stimulation took place through light-stimulating glasses (GS030-Medical Device (GS030-MD)). Previously, this methodology had been tested in the non-human primate model. What sounds like science fiction could make blind people sighted in the future. Read more in today's article.1-9
What exactly is optogenetics?
In optogenetics, cells are modified by means of light. In this way, neuronal networks can be influenced. The term optogenetics comes from the fact that light-dependent ion channels can be specifically switched on or off by light. The genetic information for these light-dependent ion channels has previously been introduced into the target cells through gene therapy. These light-dependent ion channels, also known as channelrhodopsins, were discovered in green algae almost 20 years ago. They consist of the photoreceptor rhodopsin and an ion channel and are cation-selective. This means that light leads to a depolarisation and thus to an activation of the neurons.10 A modification of these channel rhodopsins led to the possibility of a hyperpolarisation and thus an inhibition of the neurons.11 A modified adenovirus was used as an optogenetic vector in the PIONEER study. This optogenetic vector encodes the light-dependent channel rhodopsin protein ChrimsonR, which is fused to the red fluorescent protein tdTomato. By means of intravitreal injection, this vector reached its target site, the foveal ganglion cells. The maximum absorption of ChrimsonR-tdTomato is at 590 nm.1
Diagnostics after the first injection
The 58-year-old patient was diagnosed with retinitis pigmentosa 40 years earlier. The blind eye received an injection of 5.0 × 1010 vector genomes of the optogenetic vector. Ophthalmological examinations and retinal imaging by optical coherence tomography, fundus colour photographs and autofluorescence images were performed before the first injection and afterwards. Follow-up was performed over the 84-week period. Intraocular inflammation was assessed using the Standardization of Uveitis Nomenclature Working Group (SUN) classification. There were no signs of intraocular inflammation after injection.1,12,13
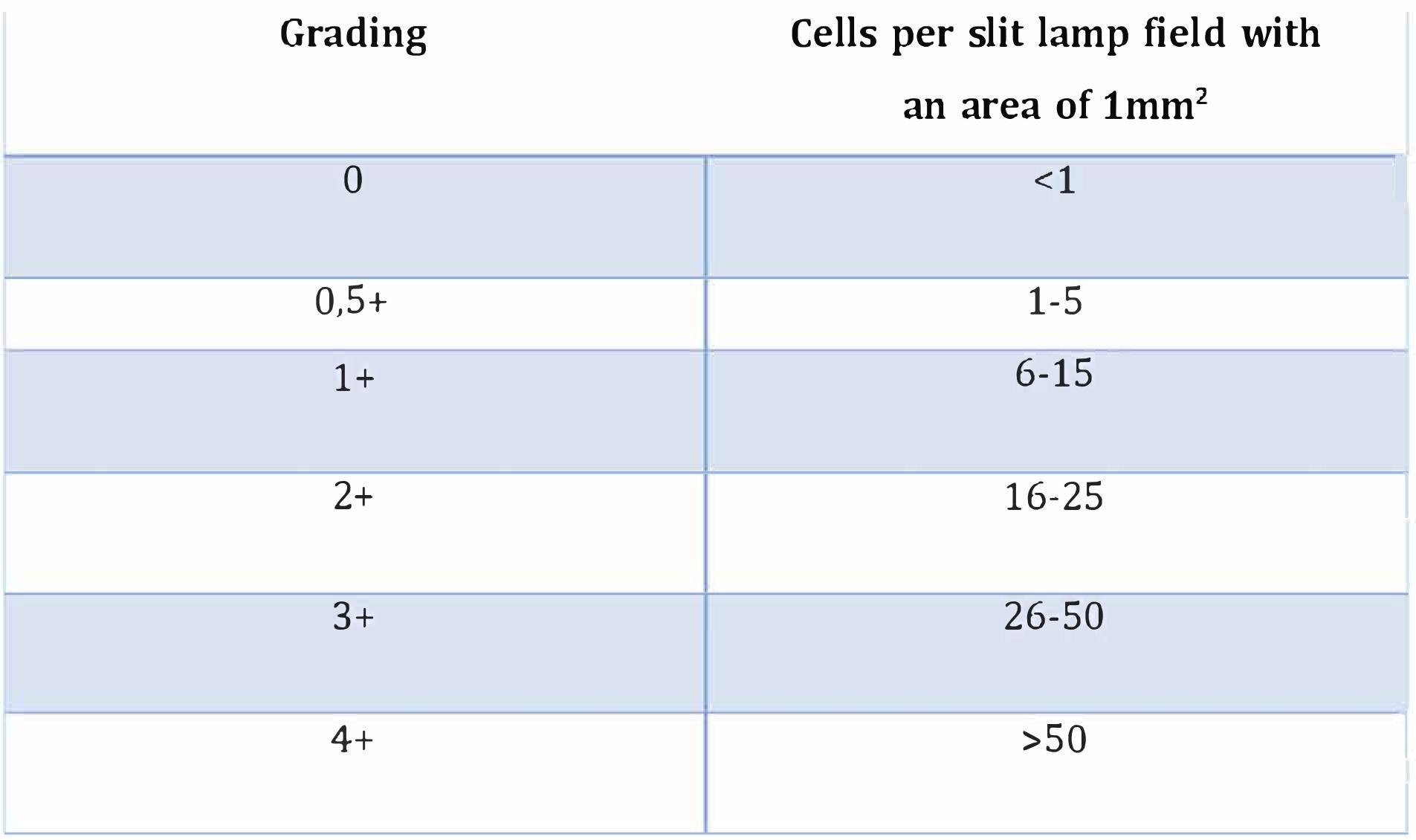
Table 1: Grade classification of anterior chamber cells according to the SUN classification.12,13
In the present recently published study, the eye that was previously blind was able to regain sight within the postoperative 84-week study period. The patient now perceived light in the treated eye. The light-stimulating glasses were tested 3 times on the patient before injection of the optogenetic vector. Before the gene therapy, the patient could not perceive light in the affected eye. Training with the light-stimulating glasses was started 4½ months after injection. This late timing was based on the following observation: In the non-human primate model, expression of ChrimsonR-tdTomato within the foveal ganglion cells was not initiated until 2 to 6 months after injection.1,8 At month 7 after starting visual training, the patient reported noticing an improvement in his vision while using the light-stimulating glasses.1
These research results give us hope that optogenetics could one day make vision possible again for people who are already blind. However, there is still a long way to go until then.
References:
1. Sahel J.A. et al. (2021). Partial recovery of visual function in a blind patient after optogenetic therapy, Nature Medicine.
2. Bi A. et al. (2006). Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron 50, 23–33.
3. Busskamp V. et al. (2010). Genetic reactivation of cone photoreceptors restores visual responses in retinitis pigmentosa. Science 329, 413–417. Lagali P. S. et al. (2008). Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat. Neurosci. 11, 667–675.
4. Roska B. et al. (2018). Restoring vision. Nature 557, 359–367.
5. Sahel J.A. et al. (2019). Depicting brighter possibilities for treating blindness. Sci. Transl. Med. 11, eaax2324.
6. Sahel J. A. et al. (2013). Gene therapy for blindness. Annu. Rev. Neurosci. 36, 467–488.
7. Scholl H. P. N. et al. (2016). Emerging therapies for inherited retinal degeneration. Sci. Transl. Med. 8, 368rv6.
8. Gauvain G. et al. (2021). Optogenetic therapy: high spatiotemporal resolution and pattern discrimination compatible with vision restoration in non-human primates. Commun. Biol. 4, 125.
9. Picaud S. et al. (2019). The primate model for understanding and restoring vision. Proc. Natl Acad. Sci. USA 116, 26280–26287.
10. Nagel G. et al.: Channelrhodopsin-1: a light-gated proton channel in green algae. Science 2002; 296: 2395–8 CrossRef MEDLINE
11. Wietek J. et al. (2014). Conversion of channelrhodopsin into a light-gated chloride channel. Science 2014; 344: 409–12 CrossRef MEDLINE.
12. Jabs D. A. et al. (2005). Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am. J. Ophthalmol. 140, 509–516.
13. Trusko B. et al. (2013). The Standardization of Uveitis Nomenclature (SUN) Project. Development of a clinical evidence base utilizing informatics tools and techniques. Methods Inf. Med. 52, 259–265.